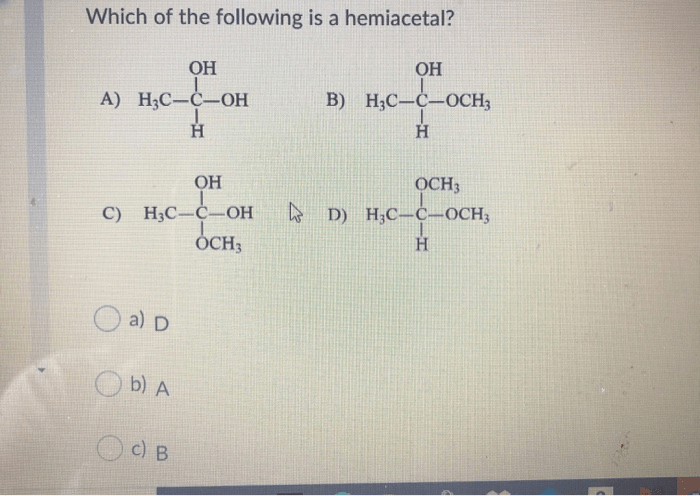
Which of the following is a hemiacetal? This question marks the commencement of an illuminating discourse on the captivating realm of hemiacetals, organic compounds that play a pivotal role in various chemical processes. Delve into this comprehensive guide as we unravel the intricacies of hemiacetals, their properties, synthesis, and diverse applications.
Hemiacetals, characterized by their unique structure and reactivity, hold immense significance in organic chemistry. Their ability to undergo further reactions makes them versatile building blocks for the synthesis of complex molecules. As we explore the world of hemiacetals, we will uncover their fascinating properties and the factors that govern their reactivity.
Hemiacetals: Which Of The Following Is A Hemiacetal
Hemiacetals are organic compounds that contain both an ether and an alcohol functional group. They are formed by the reaction of an aldehyde or ketone with an alcohol.
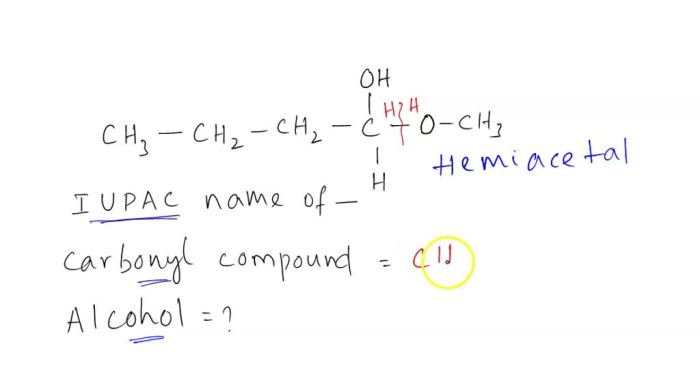
The general structure of a hemiacetal is R 1R 2C(OH)OR 3, where R 1and R 2are alkyl or aryl groups and R 3is an alkyl or aryl group.
Hemiacetals are typically formed in aqueous solutions. The reaction is reversible, and the equilibrium constant for the formation of a hemiacetal depends on the nature of the aldehyde or ketone and the alcohol.
Hemiacetals are weak acids. They can be protonated by strong acids to form oxonium ions.
Hemiacetals are also nucleophilic. They can react with electrophiles to form acetals.
Properties of Hemiacetals
Hemiacetals are typically colorless liquids or solids. They are soluble in water and organic solvents.
Hemiacetals have a characteristic IR absorption band at around 1740 cm -1. This band is due to the C=O stretching vibration.
Hemiacetals are relatively unstable compounds. They can easily hydrolyze to form the corresponding aldehyde or ketone and alcohol.
Synthesis of Hemiacetals
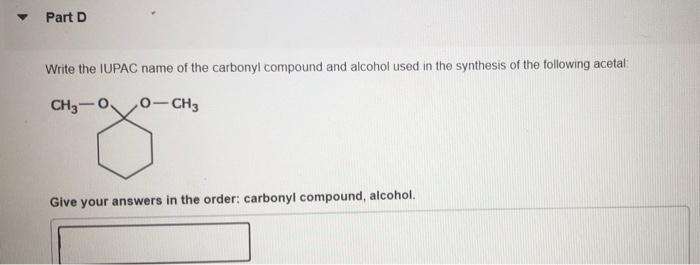
Hemiacetals can be synthesized by the reaction of an aldehyde or ketone with an alcohol. The reaction is typically carried out in an aqueous solution.
The yield of the reaction depends on the nature of the aldehyde or ketone and the alcohol. Aldehydes are more reactive than ketones, and primary alcohols are more reactive than secondary alcohols.
Hemiacetals can also be synthesized by the reaction of an epoxide with an alcohol.
Applications of Hemiacetals, Which of the following is a hemiacetal
Hemiacetals are used in a variety of organic reactions. They are used as protecting groups for aldehydes and ketones. They are also used as intermediates in the synthesis of acetals and ketals.
Hemiacetals are also used in the pharmaceutical and food industries.
Questions and Answers
What is the key structural feature of a hemiacetal?
A hemiacetal is characterized by the presence of a hydroxyl group (-OH) and an alkoxy group (-OR) attached to the same carbon atom.
How are hemiacetals formed?
Hemiacetals are typically formed by the reaction of an aldehyde or ketone with an alcohol in the presence of an acid catalyst.
What are the key physical properties of hemiacetals?
Hemiacetals are generally colorless liquids or solids with relatively low boiling points. They are soluble in water and organic solvents.